Injectable Viscobeads Technique for an Experimental Glaucoma Model
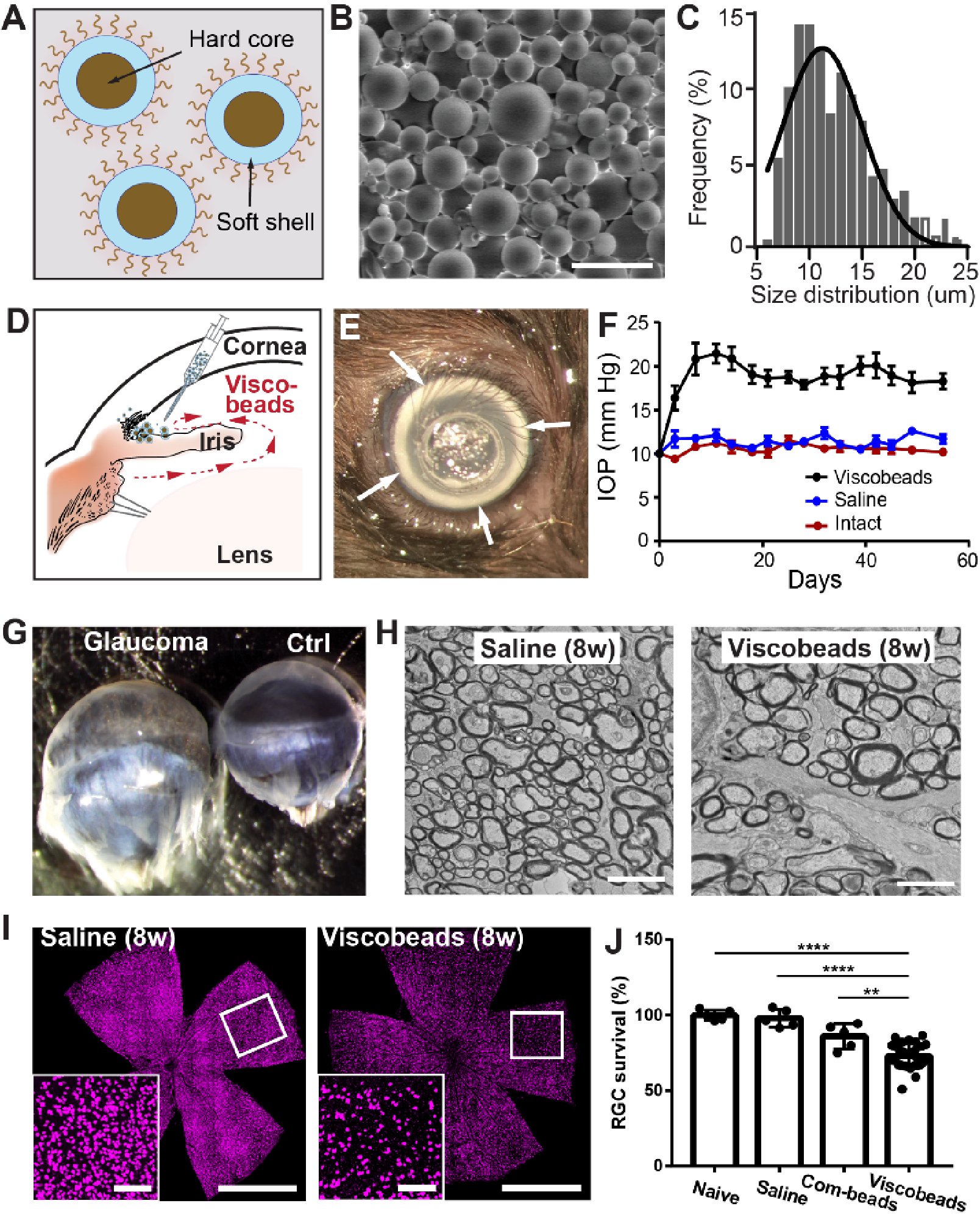
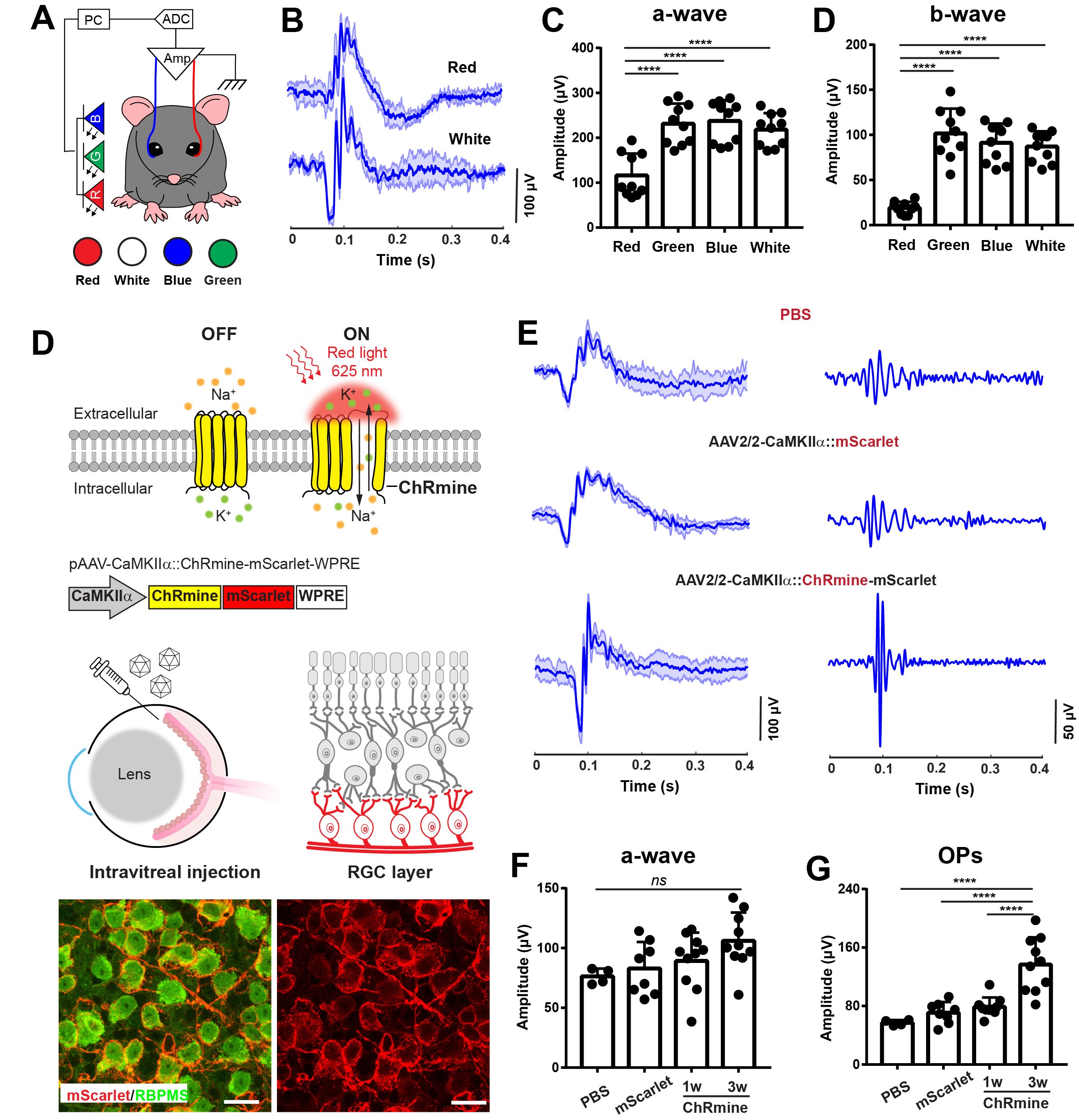
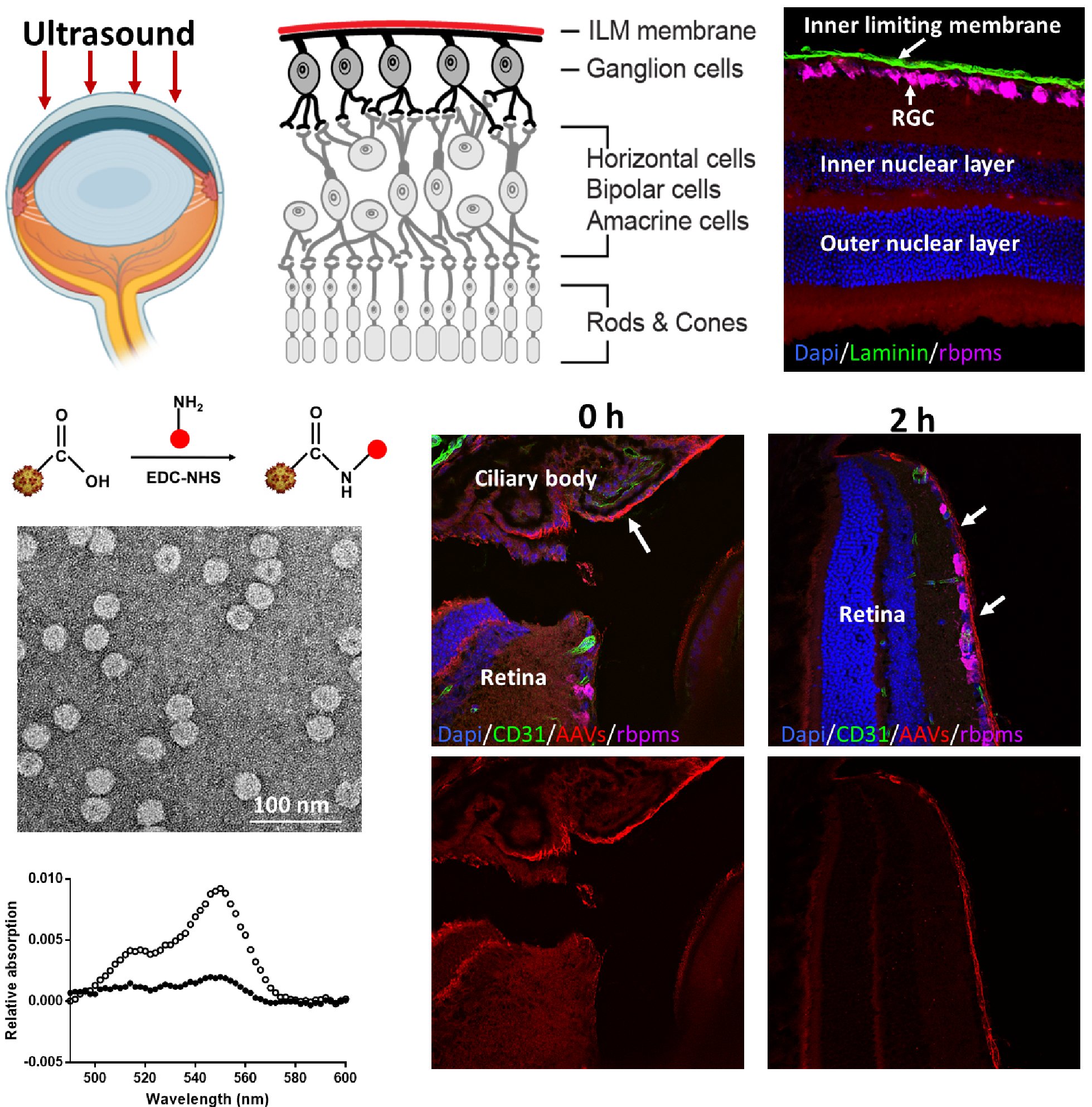
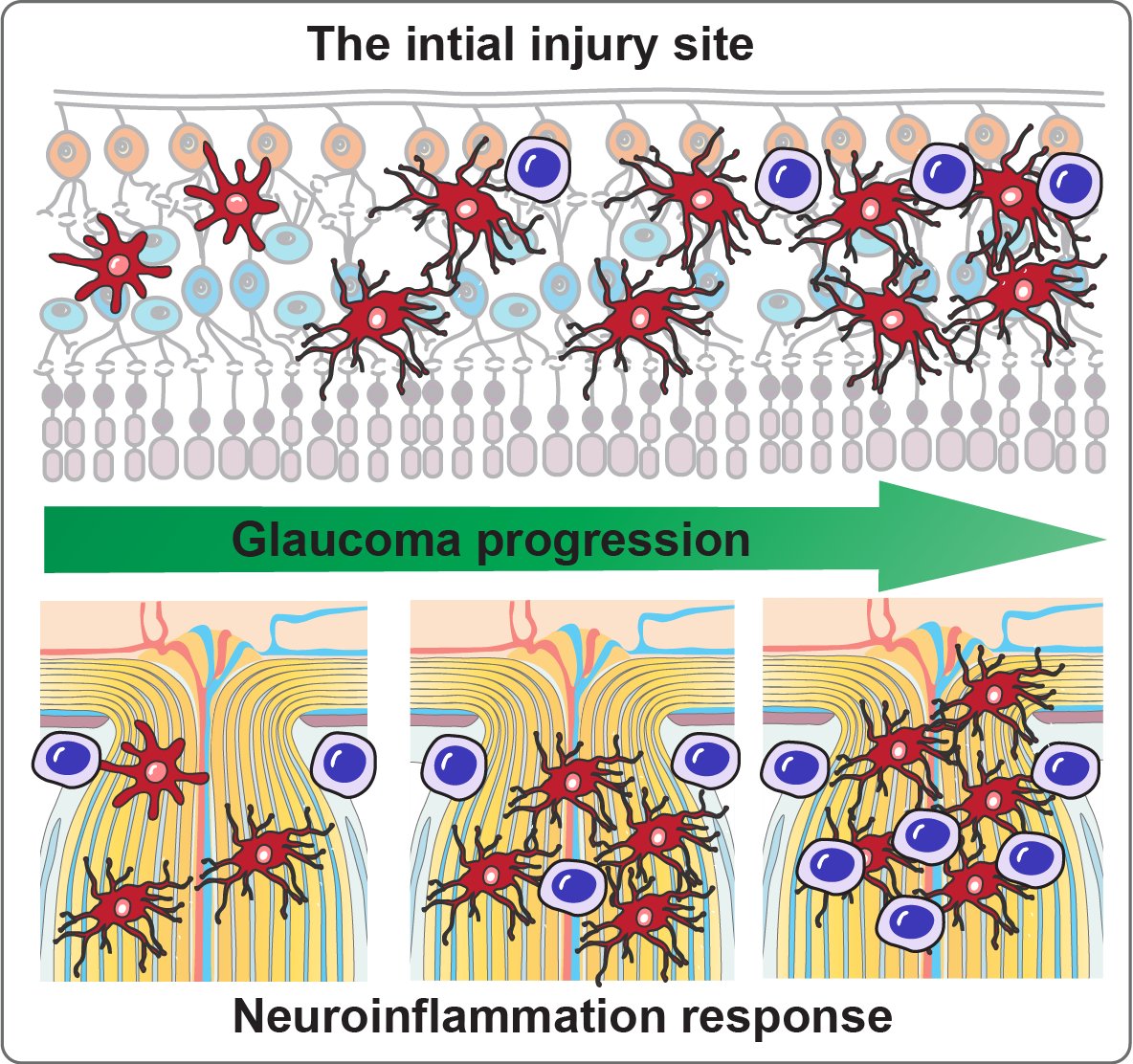
Current microbead occlusion models for glaucoma research exhibit significant variability and require multiple injections to maintain long-term ocular hypertension. The rigidity of polymeric beads and their mismatched size with trabecular meshwork (TM) structures often lead to insufficient stability of beads-tissue contact and unsatisfactory retention in TM tissues. To address these challenges, we have developed polymeric viscobeads with self-produced viscoelastic materials and a heterogeneous size distribution. This technique provides an accessible and versatile in vivo platform, facilitating the study of neurodegenerative mechanisms underlying glaucomatous progression and holding promise for future translational research applications.
To request the viscobeads sample, please contact Dr. Wang at qbwang@binghamton.edu.